Uranium
Uranium
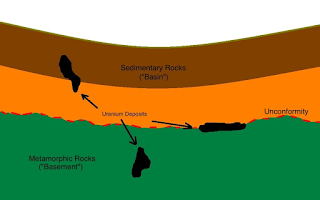
Uranium is a chemical element with symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly radioactive because all isotopes of uranium are unstable, with half-lives varying between 159,200 years and 4.5 billion years. The most common isotopes in natural uranium are uranium-238 and uranium-235. Uranium has the highest atomic weight of the primordially occurring elements. Its density is about 70% higher than that of lead, and slightly lower than that of gold or tungsten. It occurs naturally in low concentrations of a few parts per million in soil, rock and water, and is commercially extracted from uranium bearing minerals such as uraninite.
In nature, uranium is found as uranium-238 (99.2739–99.2752%), uranium-235 (0.7198–0.7202%), and a very small amount of uranium-234 (0.0050–0.0059%). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million years, making them useful in dating the age of the Earth.
Many contemporary uses of uranium exploit its unique nuclear properties. Uranium-235 is the only naturally occurring fissile isotope, which makes it widely used in nuclear power plants and nuclear weapons. However, because of the tiny amounts found in nature, uranium needs to undergo enrichment so that enough uranium-235 is present. Uranium-238 is fissionable by fast neutrons, and is fertile, meaning it can be transmuted to fissile plutonium-239 in a nuclear reactor. Another fissile isotope, uranium-233, can be produced from natural thorium and is also important in nuclear technology.
 |
| Uranium |
* Because uranium decays by alpha particles, external exposure to uranium is not as dangerous as exposure to other radioactive elements because the skin will block the alpha particles. Ingestion of high concentrations of uranium, however, can cause severe health effects, such as cancer of the bone or liver. Inhaling large concentrations of uranium can cause lung cancer from the exposure to alpha particles. Uranium is also a toxic chemical, meaning that ingestion of uranium can cause kidney damage from its chemical properties much sooner than its radioactive properties would cause cancers of the bone or liver.
Uranium 238 (U238 )
U238 is the most common isotope of uranium found in nature. It is not fissile, but is a fertile material: it can capture a slow neutron and after two beta decays become fissile plutonium-239. U238 is fissionable by fast neutrons, but cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of U-238's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control.
Around 99.284% of natural uranium is uranium-238, which has a half-life of 1.41×1017 seconds (4.468×109 years, or 4.468 billion years).[1] Depleted uranium has an even higher concentration of the 238U isotope, and even low-enriched uranium (LEU), while having a higher proportion of the uranium-235 isotope (in comparison to depleted uranium), is still mostly 238U.
U238 (Uranium-235)
Uranium metal highly enriched in uranium-235 Characterize:
Name, symbol: Uranium-235,235U
Neutrons: 143
Protons: 92
Nuclide data Natural abundance: 0.72%
Half-life:703,800,000 years
Parent isotopes: 235Pa 235Np 239Pu
Decay products: 231Th
Isotope mass: 235.0439299 u
Spin: 7/2−
Excess energy: 40914.062 ± 1.970 keV
Binding energy: 1783870.285 ± 1.996 keV
Decay mode Decay energy
Alpha: 4.679
MeV Uranium-235 is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a fission chain reaction. It is the only fissile isotope that is a primordial nuclide or found in significant quantity in nature. U-235 can be concentrated in a process called “enrichment,” making it suitable for use in nuclear reactors or weapons. Uranium235 has a half-life of 703.8 million years. It was discovered in 1935 by Arthur Jeffrey Dempster. Its (fission) nuclear cross section for slow thermal neutrons is about 504.81 barns. For fast neutrons it is on the order of 1 barn. Most but not all neutron absorptions result in fission; a minority result in neutron capture forming uranium-236. Heavy water reactors, and some graphite moderated reactors can use unenriched uranium, but light water reactors must use low enriched uranium because of light water's neutron absorption. Uranium enrichment removes some of the uranium-238 and increases the proportion of uranium-235. Highly enriched uranium, which contains an even greater proportion of U-235, is sometimes used in nuclear weapon design.
 |
| Weapon grade uranium production process |







Comments
Post a Comment