Geochemically abundant and scarce metals
Geochemically abundant and scarce metals
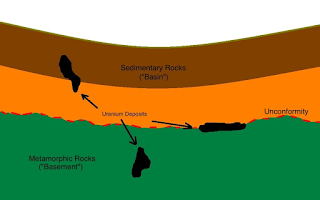
Industrial and technological Metals applications can be divide into two categories based on their frequency of existence in the Earth's crust. Abundant geochemical metals, which are five elements (aluminium, iron, magnesium, manganese, and titanium), make up more than 0.1 percent of the earth's crust, while geochemical scarce metals, which include other metals as well (Including copper, lead, zinc, gold and silver), account for less than 0.1%. Almost in every rock, by accurate chemical analysis, at least small amounts of all metals can be detected. However, there are important differences in how abundant and rare metals occur in ordinary rocks. Geochemically abundant metals are present as basic constituents in minerals. Basalt, for example, is a common igneous rock composed mainly of the olivine and pyroxene (iron-magnesium silicate) minerals, feldspar (sodium-calcium-aluminium silicate), and ilmenite (iron titanium oxide). Accurate chemical analysis of basalt, a typical rock, reveals the presence of many geochemically rare metals, but no search indicates the presence of one or more rare minerals as basic constituents.
Geochemical scarce metals rarely form minerals in ordinary rocks. Instead, they are transported through the process of atomic replacement in the structure of conventional rock-forming minerals (most of them silicates). This process involves the atomic replacement of a mineral by a foreign atom with the same ionic radius and valence, without changing the atomic bonding of the host mineral. For example, copper, zinc, and nickel atoms can replace iron and magnesium atoms in olivine and pyroxene. However, because the replacement of foreign atoms produces tensions in the atomic bonding, there are limitations to this process, which are determined by temperature, pressure, and various chemical parameters. In fact, most rare metal substitutions in conventional silicate ores are low - in many cases only a few hundred atoms per million host atoms - but even these limitations rarely exceed in ordinary rocks.
An important conclusion that emerges from the presence of rare and abundant metals in common rocks is that mineral ores of abundant metals can be found in many common rocks, while rare metal ores are only found in very few cases where some specific geological processes and local enrichment involved in which the limit of atomic replacement is exceeded.
 |
| geochemically scarce metals |







Comments
Post a Comment